Resources
Articles
Understanding Magnesia Brick Compositions
A combination of four articles will go over magnesium the element and the production of magnesia refractory products. This is Part 2 Subtle role of Calcium in Magnesia Refractories
Part 1 Magnesium the Element
Part 3: Magnesium Oxide Refractories
Part 4: Magnesia with Various Spinels
Mineralogy of Basic Refractory Brick
The mineralogy of basic refractory brick based on the subtle role of calcium. Melts can occur in alumina refractories that are glasses. Glasses, however, do not form in basic refractories, but their impurities are termed minerals. The silicate minerals determine the refractoriness of the basic brick composition.
There are five principle silicate minerals in the basic brick system. As you go down this list, the lime content of the minerals increases. The first mineral is fosterite, two magnesias combined with a silica molecule. Fosterite melts at high temperatures. If you take fosterite and add lime to it, the next mineral you reach is called monticellite. The presence of lime in this mix causes melting to start at a much lower temperature. By adding more lime, merwinite forms, which are 3 limes, one magnesia, and two silicas.
Merwinite, which is the lowest melting calcium-magnesium-silica mineral. If you have monticellite and merwinite in your brick composition, you must keep their total amount low to maintain good properties. The last two minerals are dicalcium and tricalcium silicate, familiar to you folks in the cement industry.
Basic Brick Melting Temperatures
This chart shows the respective melting temperatures, and more importantly, the mineral phase assemblages present in burned magnesite brick. As it turns out, the minerals present are defined by the ratio of the lime to the silica. Silica is 60 grams per mole, and lime is 56 grams per mole. Simple division of the lime content by the silica content will get us close enough. At a lime to silica ratio below 0.93, magnesia (M) co-exists with fosterite, abbreviated M2S.
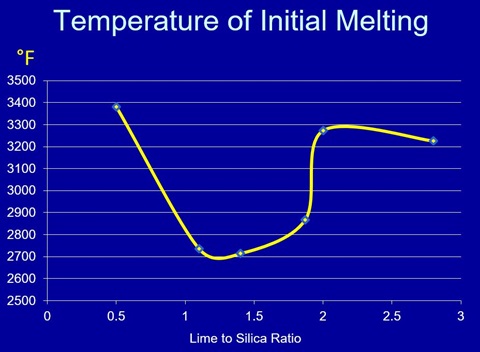 Fosterite has a melting temperature of 3380F, so this is a very refractory combination. If we increase the lime content that the lime to silica ratio is between 0.9 and 1.4, the mineral phases are now magnesia. These include monticellite (CMS), and merwinite (C3MS2). With the lowest melting temperature of 2714°F.
Fosterite has a melting temperature of 3380F, so this is a very refractory combination. If we increase the lime content that the lime to silica ratio is between 0.9 and 1.4, the mineral phases are now magnesia. These include monticellite (CMS), and merwinite (C3MS2). With the lowest melting temperature of 2714°F.
Increased lime in the system, such that our ratio is above 1.87, which is actually 2:1 lime and silica on a molar basis. We now have magnesia and dicalcium silicate (C2S) co-existing and with higher lime contents, tricalcium silicate (C3S) joins the mix. This is now a very refractory system. Remember, of course, that these are melting temperatures in pure systems, and that in industry, we almost never have pure systems. As we will find out later, there are combinations of magnesia and spinels which introduce several other minerals into the system. those additions can bring eutectics or other points of lower melting.
This is a graph of the temperatures of initial melting for the magnesia brick system with the various ratios of lime and silica. Note the decrease in refractoriness between about 0.9 and 2.
Dead Burned Magnesia Grades
The 98-grade synthetic has a lime to silica ratio of about 1.35. This suggests the initial melting temperature is going to be around 2700°F. This grade of magnesia, however, has been used to produce direct-bonded brick at temperatures much higher than the 2700°F. An important factor to consider is the total lime and silica content.
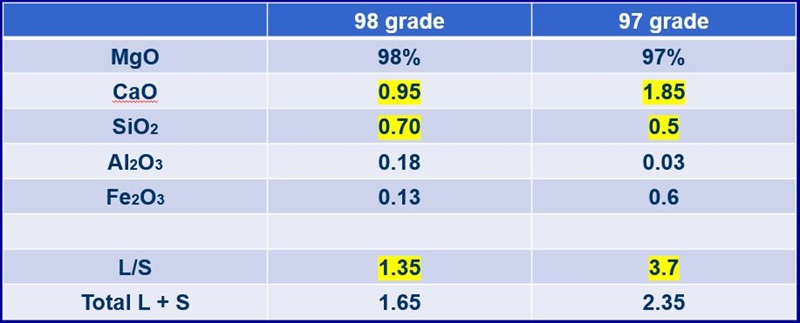
This magnesia has about just over 1.5% total lime and silica. If the mineral phases will have low temperatures of initial melting, then it is good to keep the total lime and silica low. Now, look at the 97-grade. The 97-grade has 40% more total lime and silica, but its ratio is over 3. This indicates that silicate mineral phases are dicalcium and tricalcium silicate.
A refractory product that is made with 97-grade may not be fired quite as high of a temperature. This is due to the greater amount of lime and silica. However, 97 will most likely make a pretty good magnesite brick.
Magnesia Brick Comparison
This chart compares the physical properties of two magnesia bricks that Resco makes, PERECON and OXILINE H. These are both used in the hearths of steel furnaces and can be used in non-ferrous metal furnaces. Of the two, PERECON has lower purity. Note total lime and silica is 3.6 percent. And its lime-to-silica ratio is about 0.9, so the silicate minerals will show initial melting at fairly low temperatures.
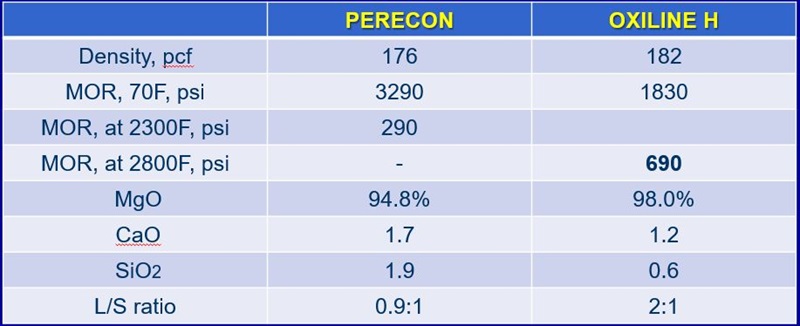
MOR stands for Modulus of Rupture, which is the flexural or bending strength of the refractory. At room temperature, PERECON is very, very strong with a MOR over 3,000 psi. This is explained by the high total lime + silica.
If we look at the hot strength, however, its MOR at 2300°F is only about 290 psi. PERECON is used in applications that are not very high temperatures, for example, copper smelting. It was engineered for hydration resistance, that is, resistance to the formation of magnesium hydroxide as a result of contact with water.
OXILINE H is also a high magnesia brick. It was engineered for an entirely different application, principally use in a basic oxygen steel converter. In a BOF, high hot strength is necessary due to the mechanical wear of charging solid steel scrap. Impact and abrasion can damage the refractory.
The hot modulus of rupture of 660 psi at 2800°F, 500 degrees higher than the temperature we test the PERECON. OXILINE H is a very strong brick at elevated temperatures. The secret is the low lime + silica content at 1.8% and the 2:1 lime to silica ratio.

Leave a comment