Resources
Articles
Understanding Magnesia and its Relation to Refractory
A combination of four articles will go over magnesium the element and the production of magnesia refractory products. This is Part 1 Magnesium the Element
Part 2: Subtle role of Calcium in Magnesia Refractories
Part 3: Magnesium Oxide refractories
Part 4: Magnesia with Various Spinels
Magnesium is a highly reactive metal. It is a good reducing agent in metallurgy. It is the second most abundant cation (that is, positive ion) in seawater, so it is pretty common on this planet. Magnesium metal has a fairly low melting temperature at just over 1200 degrees-F. It is also a co-factor in over 300 enzyme systems in the human body.
Magnesium Metal Production
The production of magnesium metal worldwide is about 1.1 million tons annually. 85% of magnesium metal is produced in China, and until recently was made primarily using the Pidgeon process. US production of magnesium is about 50,00 tons. In the US, magnesium is produced electrolytically with a salt feed from the Great Salt Lake in Utah. China dwarfs the rest of the world in magnesium production.
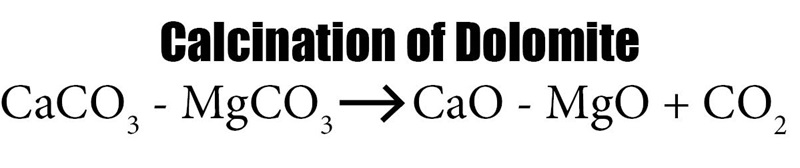
Magnesium Production by Pidgeon Process
80% of global magnesium production is by the Pidgeon Process. The Pidgeon Process was invented by Dr. Lloyd Montgomery Pidgeon, and the first plant operated in 1941 in Timmins, Ontario. It is also called the silico-thermic reduction of calcined dolomite. The source of silicon metal is ferrosilicon, and the final product magnesium is distilled off in a vacuum and collected.
This is a solid-state reaction. Interesting note, China has recently enacted some restrictions on air emissions from its magnesium plants. Roskill, a trade organization that follows metallurgical production, says the following: “Increasingly stringent environmental regulations in China have already caused the closure of several plants using the Pidgeon Process and more will probably shutter."
Magnesium metal can also be manufactured electrolytically. This is referred to as the Dow Process. In 1940, Dow Chemical built a plant in Texas to produce magnesium for lightweight aircraft parts from seawater and dolomite. The initial step involves dissolving dolomitic lime in brine or seawater, causing magnesium hydroxide (in yellow) to precipitate out. The magnesium hydroxide is then filtered and treated with hydrochloric acid to produce magnesium chloride. Then sent to a dryer where it becomes the feed for the electrolysis cell that produces the magnesium metal.
Today, the primary uses of magnesium metal are for aluminum alloys and die-casting. The magnesium metal is alloyed with zinc prior to making the castings. Magnesium metal is also used in the desulfurization of steel. The fourth use of magnesium is as the reducing agent in titanium production by the Kroll process.
In North America, some new construction has been announced in the magnesium industry. Recently ground was broken for a new plant by Alliance Magnesium of Asbestos, PQ. Feedstock for the plant will be tailings from the asbestos industry. As you know, Quebec has good, low-cost hydroelectric power.
Magnesium Oxide
Magnesium oxide is an ionic solid. Your daily contact with magnesium oxide may be in our kitchen in the form of electrical range-top heating elements. This can be found where the two properties of high thermal conductivity and low electrical conductivity make it an ideal material for the task. Magnesium oxide, in its crystalline form, takes on the cubic crystal structure of halite – salt.
56% of magnesia produced worldwide is for refractories in the form of dead-burned magnesite or fused magnesite. View Magnesite Brick. Other uses are agricultural including animal feed, chemical uses for the neutralization of acids, construction, and environmental.
Magnesium Oxide Process
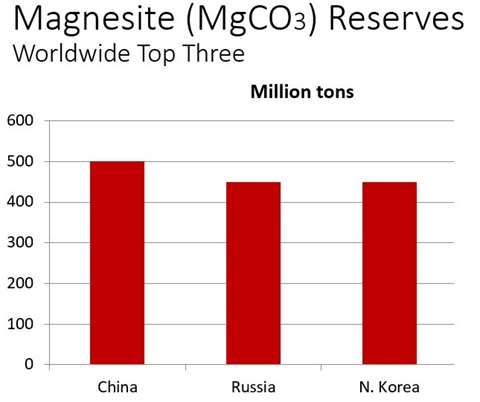 The simplest way to get magnesia is through the high-temperature calcination of magnesium carbonate, called magnesite ore. This reaction is analogous to the calcination of limestone to get lime. Unfortunately, you are at the mercy of nature, in that any impurities in the magnesite deposit, for example, calcium, iron oxide, alumina, or silica, get carried over in the magnesia product. The three largest reserves of magnesite ore, in million tons, are China, Russia, and North Korea.
The simplest way to get magnesia is through the high-temperature calcination of magnesium carbonate, called magnesite ore. This reaction is analogous to the calcination of limestone to get lime. Unfortunately, you are at the mercy of nature, in that any impurities in the magnesite deposit, for example, calcium, iron oxide, alumina, or silica, get carried over in the magnesia product. The three largest reserves of magnesite ore, in million tons, are China, Russia, and North Korea.
Magnesia producers in China received notice that open-pit magnesite mining using explosives must be halted started July 1, 2019. This lasted for half a year to reduce air pollution and relieve high magnesia inventories. Resulting in falling exports of magnesia from China.
Magnesia Compositions
Magnesia can also be prepared synthetically. The starting point is calcined dolomitic lime, which is reacted with brine which causes magnesium hydroxide to precipitate out of solution. The magnesium hydroxide is then filtered to remove excess water and calcined to form magnesia.
Chemical grade, or caustic calcined magnesia products, which need to be reactive, are calcined at temperatures at or below 1800F. Refractory grade dead-burned magnesia is sintered in kilns between 2800°F to 3600°F. Fused magnesia is formed by melting in an electric furnace at over 5,000°F.
Dead-burned magnesia is abbreviated DBM. The crystalline form of magnesia is called periclase, whose melting point is 5,070°F.
This chart shows two commercial grades of dead burned magnesia. The 98-grade is a synthetic grade, made from deep well brines. The overall impurity level is quite low. The 97-grade on the right is a natural DBM from magnesite ore. Note the higher lime and the iron oxide levels.
Fused magnesia is produced in an electric furnace. The primary refractory application is for slag-resistant magnesite-carbon brick for steel furnace and steel ladle linings. Due to substantially higher energy costs, fused magnesia is normally much more expensive than dead-burned magnesia. Fused grains are much more slag resistant than dead-burned sintered grains. Fused grains are less easily wetted by molten slags. They also have a lower solubility in melts and have slower kinetics of the reaction. These features make them often preferred in metal-making furnaces.

Leave a comment